About Sandoz® Treprostinil Injection
The first fully substitutable generic for Remodulin® (treprostinil) Injection1,2
Available to treat patients with pulmonary arterial hypertension (PAH; WHO Group 1), Treprostinil Injection offers a known treatment option at a lower price.1,3* Treprostinil Injection is the first FDA-approved generic for Remodulin®.1,2
A therapeutic equivalent for a known molecule2-7
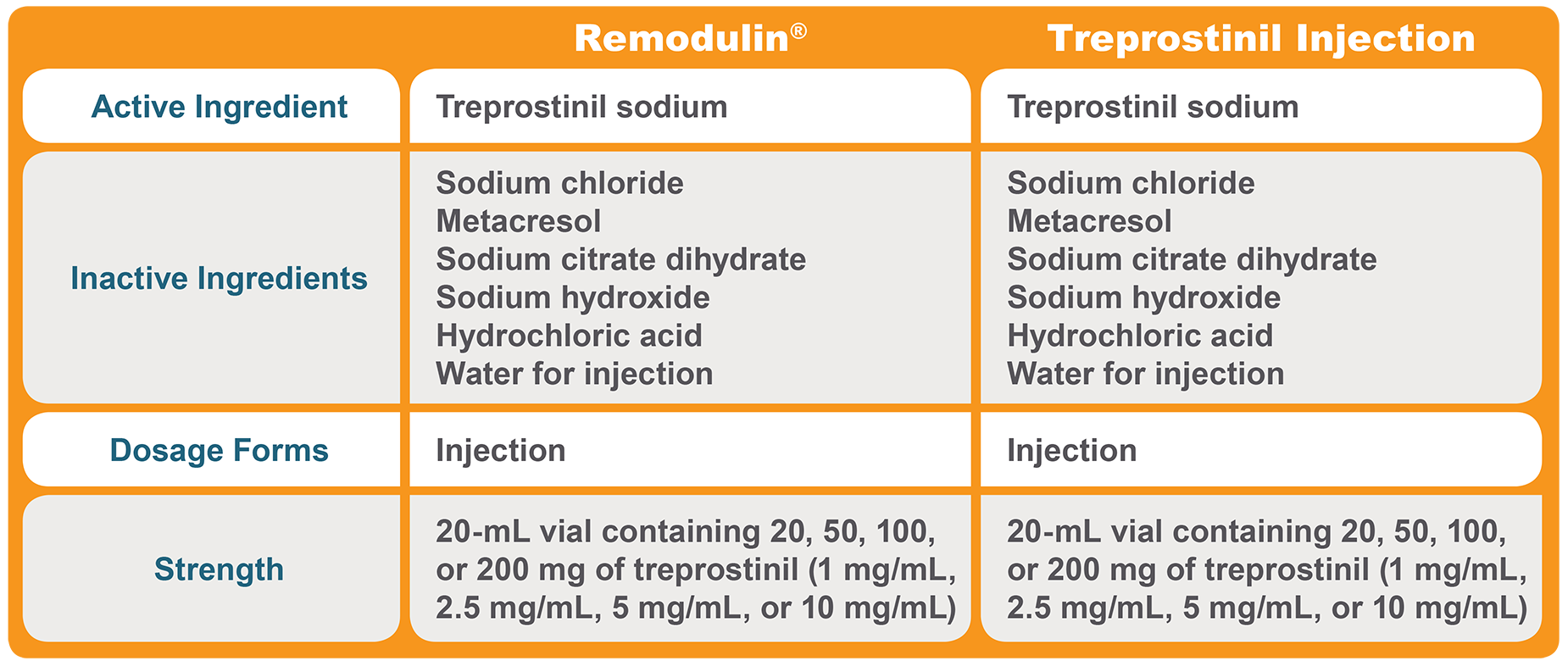
Treprostinil Injection is therapeutically equivalent to Remodulin® 1,3,4,7
| • | Same active ingredient |
| • | Same strengths |
| • | Same dosage forms |
Treprostinil Injection is qualitatively and quantitatively equivalent to Remodulin® 1-4,7
| • | Same inactive ingredients |
| • | Quantitatively the same inactive ingredient amounts |
*Sources: AnalySource Online and Red Book Online.
Therapeutic Equivalence1,3,4
Therapeutic Equivalence: Formulation1,3,4
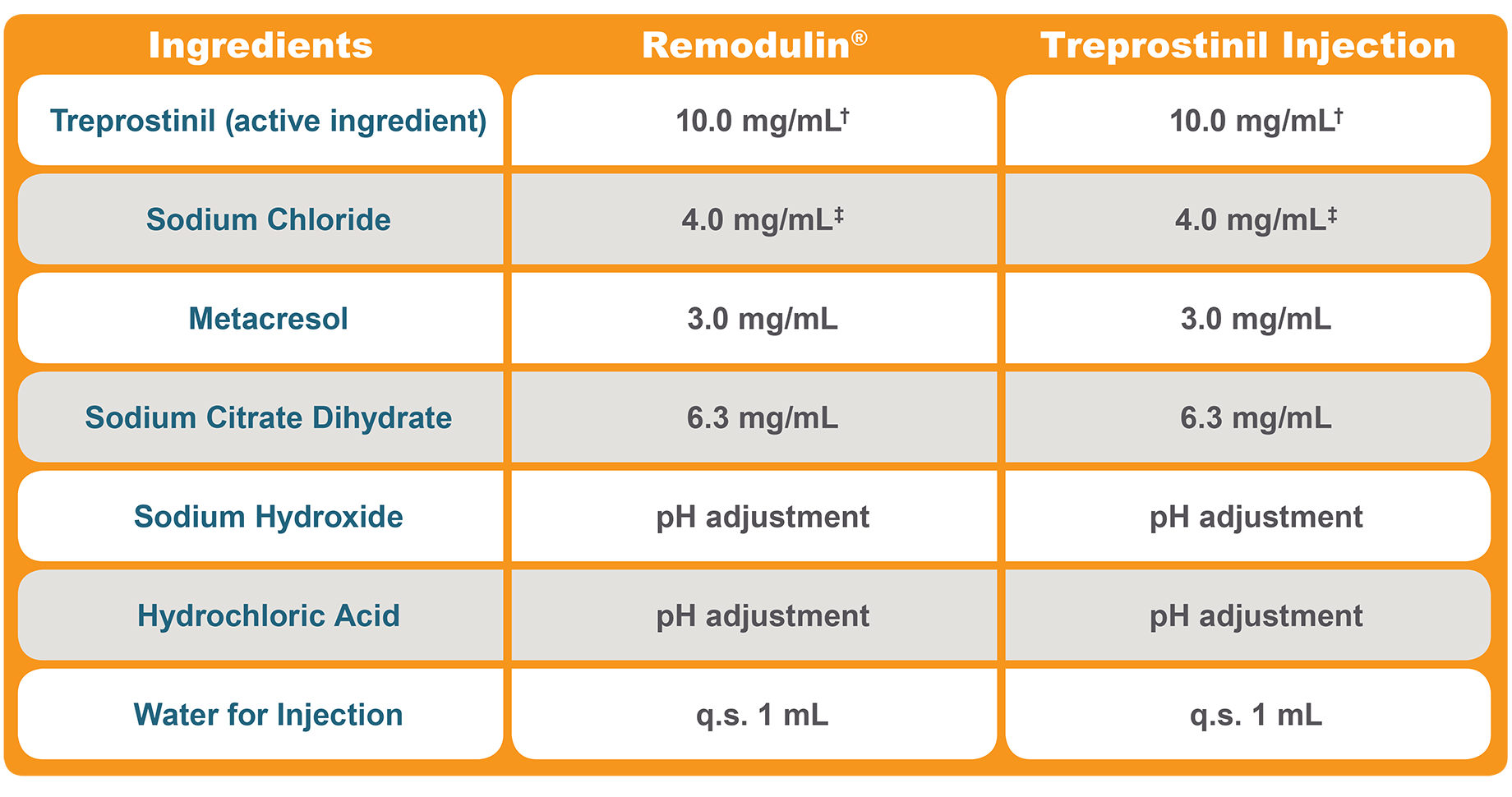
†Treprostinil Injection and Remodulin® are both available in strengths of 1, 2.5, 5, and 10 mg/mL.3,4
‡Each mL also contains 5.3 mg sodium chloride (except for the 10-mg/mL strength, which contains 4.0 mg sodium chloride).3,4

Supplied by Sandoz
| • | A global leader in generics and biosimilar medicines |
| • | With a supply chain providing trusted quality and supply |
Commercial oversight and market support provided by Liquidia
| • | A team with deep experience in PAH |

References: 1. Data on file, Sandoz Inc. 2. Drugs@FDA: FDA Approved Drug Products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=203649. Accessed October 16, 2018. 3. Treprostinil Injection [package insert]. Princeton, NJ: Sandoz Inc; April 2023. 4. Remodulin [package insert]. Research Triangle Park, NC: United Therapeutics Corp; 2021. 5. Orenitram [package insert]. Research Triangle Park, NC: United Therapeutics Corp; 2021. 6. Tyvaso [package insert]. Research Triangle Park, NC: United Therapeutics Corp; 2022. 7. Office of Generic Drugs. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. 38th ed. Rockville, MD: U.S. Dept. of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Pharmaceutical Science, Office of Generic Drugs; 2018:iv-ADB1. https://www.fda.gov/downloads/drugs/ developmentapprovalprocess/ucm071436.pdf. Accessed October 16, 2018.
