Cost Savings
Today's PAH treatment options can carry a significant financial burden for patients

Driving down costs with Sandoz® Treprostinil Injection
Sandoz Treprostinil Injection, for subcutaneous (SC) or intravenous (IV) use is a generic medicine that is therapeutically equivalent to the brand name medicine, Remodulin® (treprostinil) Injection.1
Since the launch of Treprostinil Injection, clinicians prescribing generic have decreased the average monthly out-of-pocket costs for Medicare Part B patients by ~$600 and decreased monthly costs allocated to CMS for Medicare Part B patients by ~$2,400.2,3

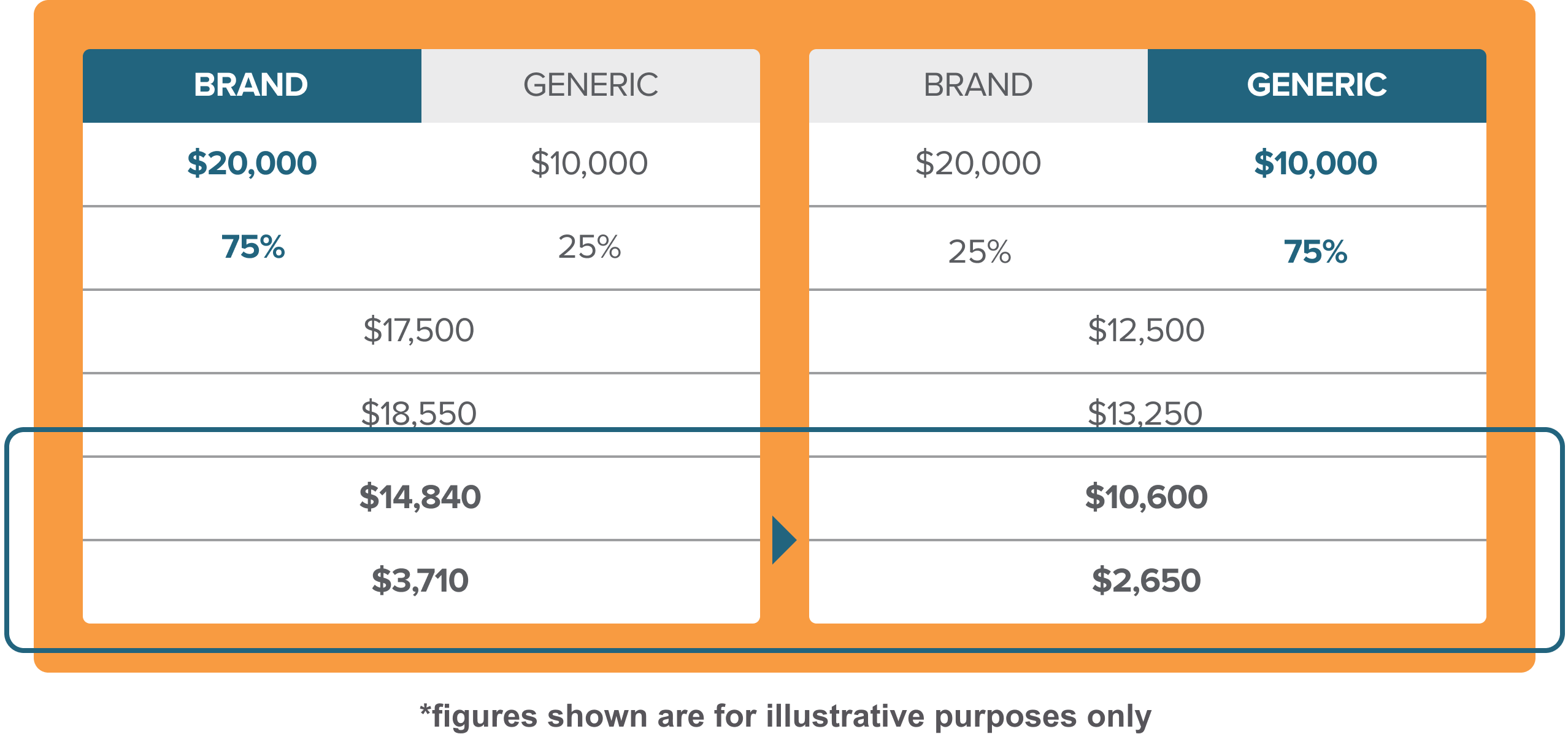
Example: Cost Impact Scenarios6
Financial implications when two products (a brand and a generic) have the same billing code
Shifting the market toward generic can potentially provide significant savings
In this example there would be >$1000 in additional monthly savings to the patient and >$4000 in additional monthly savings to CMS
- Manufacturer ASP
- Market Share
- Shared J-Code ASP
- Shared J-Code ASP +6%
- CMS Cost Share = 80%
- Patient Cost Share = 20%
It’s in your hands
By prescribing generics, such as Sandoz® Treprostinil Injection, in place of brand name medications, you can help patients and the healthcare system achieve significant savings.
The only thing generic about Sandoz® Treprostinil Injection is the price
- The same specialty pharmacies that dispense, and provide patients with PAH-related support services, for brand Remodulin® (treprostinil) (Accredo)
- Prescribers and clinics supported by Liquidia’s PAH experienced sales force
- Trusted supply and quality provided by Sandoz
- Available for intravenous (IV) and subcutaneous (SC) administration
